GRATA RERUM NOVITAS CAR-T: The Two Faces of Our Immune System
CAR-T cells constitute a type of treatment in which a patient’s T-cells (a type of immune cell) are modified in the laboratory with the objective of them binding to and destroying cancer cells. These cells are obtained thanks to the following process: the blood from a vein in the patient’s arm flows through a tube into an apheresis machine, which removes the white blood cells, including the T cells, and sends the rest of the blood back to the patient. Then, the gene for a special receptor called chimeric antigen receptor (CAR) is inserted into the T-cells in the laboratory. Millions of CAR-T cells are grown in the lab and then given to the patient by infusion. CAR T cells can bind to an antigen on cancer cells and kill them (Figure 1).

Figure 1. Diagram representing the generation of CAR-T cells.
Chimeric antigen receptor T-cell therapy (CAR-T) has been revolutionary, since it has produced remarkably effective and durable clinical responses. CARs are engineered synthetic receptors that can redirect lymphocytes, or T cells, to recognise and eliminate cells expressing a specific target antigen. The CAR binding to target antigens expressed on the cell surface is independent of the MHC receptor, resulting in vigorous T-cell activation and potent anti-tumour responses.
The unprecedented success of anti-CD19 CAR-T cell therapy against B-cell malignancies has led to the approval of a total of 6 drugs by the European Medicines Agency (EMA) (Table 1).
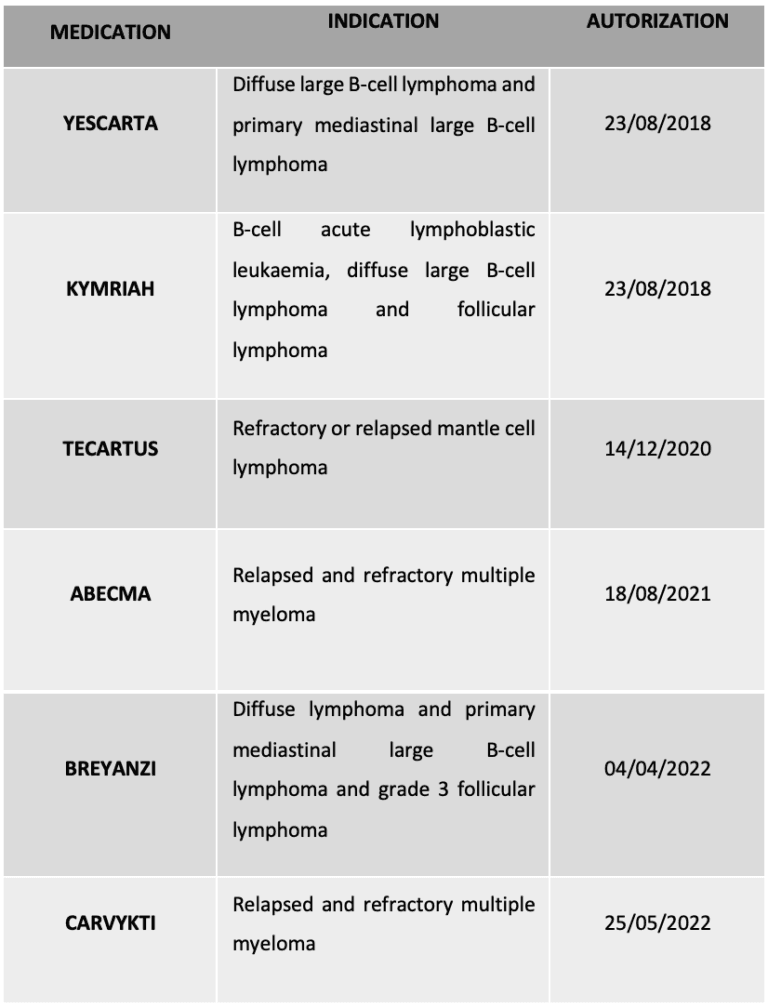
Table 1. CAR-T therapies currently approved by the EMA
However, there are important limitations to CAR-T cell therapy that remain unresolved, including life-threatening CAR-T cell-associated toxicities, limited efficacy against solid tumours, inhibition and resistance in B-cell malignancies, antigen escape, limited persistence, poor trafficking and tumour infiltration, and the immunosuppressive microenvironment. In addition, the personal must adapt to meet the needs of this growing and evolving field by developing educational training programmes.
Many approaches have been proposed, including combining CAR-T cell therapy with other cancer therapies or employing innovative CAR engineering strategies to improve anti-tumour efficacy, expand clinical efficacy and limit toxicities. In terms of potential side effects, the most common are diarrhoea, vomiting, headache, dizziness, and weakness. More serious ide effects include neurotoxicity or the widely known cytokine storm, both of which result from the operation of this type of gene therapy, triggering a systemic inflammatory process.
Regarding these side effects, it should not be forgotten that at the end of 2023, the US Food and Drug Administration (FDA) issued a ‘serious risk’ warning after receiving 19 reports of T-cell malignancy in treated patients. This would affect all six CAR-T-based therapies approved to date (Table 1), but still, experts warn that this is a standard safety procedure, and the benefits far outweigh the potential problems. Therefore, at a national level, we have researchers and teams of professionals who are already looking to take the technique a step further. Their goal: to enhance the versatility and advantages of CAR-T cells while minimising their limitations.
An example of this national effort, is an ambitious project with researchers from the Spanish National Research Council (CSIC), that will develop biological capsules that will hold CAR-T cells to more effectively and specifically treat solid tumours. Like a microscopic Trojan horse, these tiny capsules or pills will conceal anti-tumour cells inside, and will be able to invade the cancerous area and kill the malignant cells from within the tumour. The project, funded by the Ministry of Science and Innovation, involves the Salamanca Cancer Research Centre (CIC, a joint research institute of the CSIC and the University of Salamanca), the Centre for Biomedical Research in Cancer Network (CIBERONC), the University of Santiago de Compostela (USC) and the Applied Medicine Research Centre (CIMA) of the University of Navarra.
Another clear example is that of researchers from the Experimental Haematology Group at the Vall d’Hebron Institute of Oncology (VHIO), which is part of the Vall d’Hebron Campus. They have participated in the development of a new technology to process CAR-T cells more quickly and efficiently, which could reduce the waiting time for patients. The results of the study in preclinical models and the preliminary results of the first clinical trial in patients with non-Hodking B lymphoma have been published in the journal Cancer Discovery.
This demonstrates Spain’s position as one of the key players in the development and optimisation of one of the most technologically revolutionary therapies of recent years.
Regulatory and applicable standards wise, CAR-Ts would be categorize as a biological medicine for human use and, more specifically, a type of Advanced Therapy, and therefore, Part IV of the Good Manufacturing Practice (GMP) regulations would apply to them. In addition, as stated in the EMA/CAT/600280/2010 Rev.1 guideline:
‘Gene therapy medicinal product means a biological medicinal product which fulfils the following two characteristics:
(a) it contains an active substance which contains or consists of a recombinant nucleic acid used in or administered to human beings with a view to regulating, repairing, replacing, adding or deleting a genetic sequence.
(b) its therapeutic, prophylactic or diagnostic effect relates directly to the recombinant nucleic acid sequence it contains, or to the product of genetic expression of this sequence.
Gene therapy medicinal products shall not include vaccines against infectious diseases.’
CAR-T cells are genetically modified T-cells and therefore considered a product of gene therapy.
Bibliography:
- De Marco R. C., Monzo H. J., Ojala P. M. CAR T Cell Therapy: A Versatile Living Drug.IJMS, 2023; 24(7), 6300. https://doi.org/10.3390/ijms24076300.
- Labanieh L., Majzner R.G., Mackall C.L. Programming CAR-T Cells to Kill Cancer. Biomed. Eng. 2018;2:377–391. doi: 10.1038/s41551-018-0235-9.
- Sterner R.C., Sterner R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7.
- Wagner D.L., Fritsche E., Pulsipher M.A., Ahmed N., Hamieh M., Hegde M., Ruella M., Savoldo B., Shah N.N., Turtle C.J., et al. Immunogenicity of CAR T Cells in Cancer Therapy. Rev. Clin. Oncol. 2021;18:379–393. doi: 10.1038/s41571-021-00476-2.
- Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B, Curran K.J., et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449–59. doi: 1056/NEJMoa1709919.
